
When a pregnant person takes a medication, it doesn’t stay in their body alone. The drug travels through the bloodstream and reaches the placenta - the lifeline between mother and baby. But here’s the thing: the placenta isn’t a wall. It’s more like a smart filter. Some drugs slip right through. Others get blocked. And the ones that make it across? They can change how the baby grows, develops, or even survives after birth.
The Placenta Isn’t a Force Field
For decades, doctors assumed the placenta protected the fetus like a shield. That changed in the late 1950s when thousands of babies were born with severe limb defects after their mothers took thalidomide for morning sickness. The drug didn’t just pass through - it wrecked developing bones. That tragedy forced medicine to rethink everything. The placenta doesn’t keep drugs out. It decides which ones get through, and why. At full term, the placenta weighs about half a kilogram, spans 15 to 20 centimeters, and has a surface area bigger than a small bedroom floor - nearly 15 square meters. That’s not just for oxygen and nutrients. It’s also the main highway for drugs. And it’s not passive. It’s packed with proteins that actively push certain drugs back toward the mother’s side. These are called efflux transporters - especially P-glycoprotein and BCRP. Think of them as bouncers at a club. If a drug looks suspicious, they kick it out before it reaches the baby.What Makes a Drug Cross?
Not all drugs are created equal when it comes to crossing the placenta. Four key traits decide if a drug makes it to the fetus:- Molecular weight: Drugs under 500 daltons (Da) slip through easily. Ethanol? 46 Da. Nicotine? 162 Da. Both cross quickly. Insulin? 5,808 Da. Almost none gets through.
- Lipid solubility: Fats love to move through cell membranes. If a drug dissolves well in fat (log P > 2), it crosses faster. That’s why many antidepressants and seizure meds get through so readily.
- Protein binding: Only the unbound part of a drug can cross. Warfarin, for example, sticks tightly to proteins in the blood. Even though it’s small and fat-soluble, less than 1% of it reaches the fetus.
- Ionization: At the body’s normal pH (7.4), drugs that are charged (ionized) struggle to pass. Non-ionized drugs, like most opioids and SSRIs, move freely.
Take sertraline, an SSRI used for depression. Its cord-to-maternal concentration ratio is 0.8 to 1.0 - meaning the baby’s blood has almost as much as the mother’s. That’s why about 30% of babies exposed to SSRIs in late pregnancy show temporary symptoms like jitteriness, feeding trouble, or breathing issues after birth. It’s not a birth defect. It’s a withdrawal reaction.
How Transporters Block or Boost Drugs
The placenta doesn’t just sit there. It fights back. P-glycoprotein and BCRP are the main defenders. They pump drugs like HIV medications - saquinavir, indinavir, lopinavir - back into the mother’s blood. Studies using human placenta models show that when these pumps are blocked, fetal drug levels can jump by 1.7 to 2.3 times. That’s huge. But here’s the twist: not all drugs are affected the same way. Lopinavir only reaches 60% of maternal levels in the fetus because of these pumps. Zidovudine, another HIV drug, uses a different route - it hitches a ride on nutrient transporters - and reaches 95% of maternal levels. That’s why doctors often choose zidovudine over other HIV drugs during pregnancy. It’s not just about effectiveness. It’s about how much the baby gets. Even painkillers like methadone and buprenorphine are caught in this tug-of-war. Methadone reaches 65-75% of maternal levels in the baby. That’s why 60-80% of infants born to mothers on methadone develop neonatal abstinence syndrome - they go through withdrawal after birth. Buprenorphine? It’s slightly better. Fetal levels are lower, and withdrawal symptoms are often milder.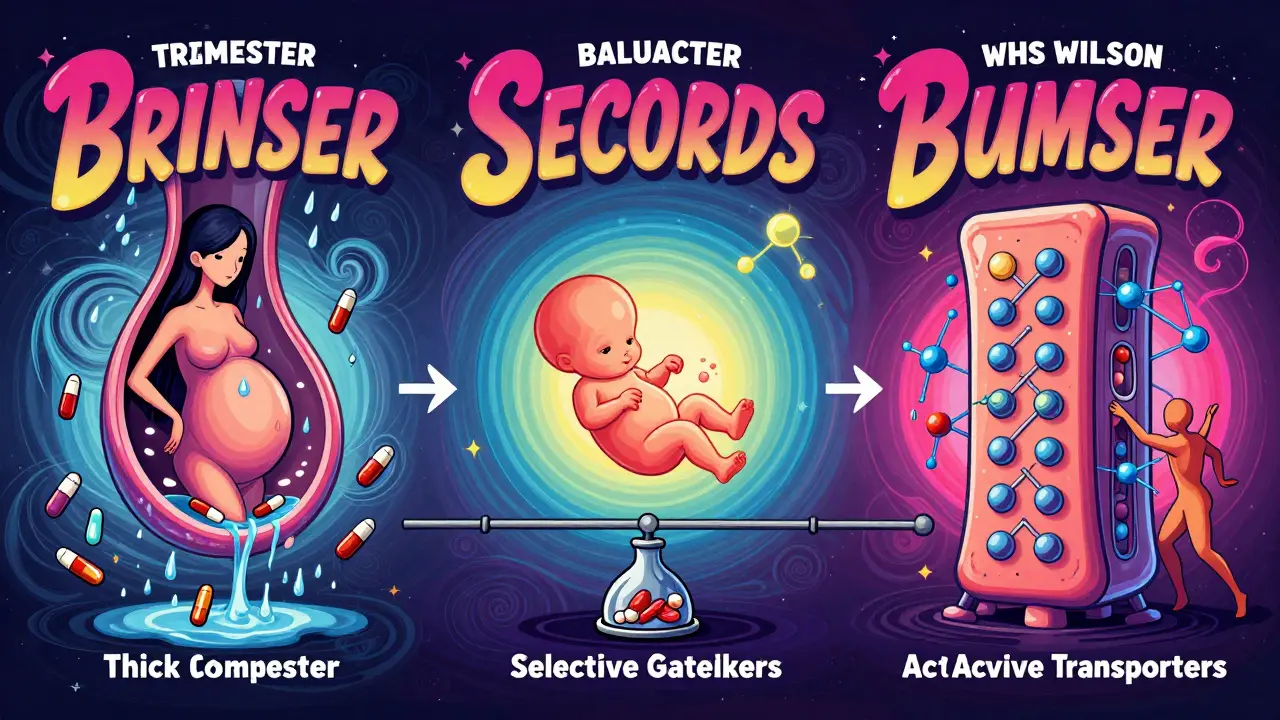
Timing Matters - First Trimester Is Riskiest
The placenta changes as pregnancy goes on. Early on, it’s leakier. Tight junctions between cells aren’t fully formed. Efflux transporters aren’t fully active. That means drugs cross more easily in the first trimester - right when organs are forming. That’s why exposure to valproic acid, an antiseizure drug, during the first 12 weeks leads to a 10-11% risk of major birth defects - like spina bifida or heart problems. In the general population? Just 2-3%. Phenobarbital, another seizure med, crosses just as easily and carries similar risks. But newer drugs like lamotrigine? Much lower transfer. That’s why doctors now prefer it. And it’s not just drugs. Alcohol crosses faster early on. So does nicotine. That’s why quitting smoking or avoiding alcohol before conception matters more than people think. Damage can happen before a woman even knows she’s pregnant.What About Chemotherapy and Other High-Risk Drugs?
Cancer treatment during pregnancy is one of the toughest decisions. Some chemo drugs cross easily. Paclitaxel? About 25-30% gets through. But if you block P-glycoprotein - say, with another drug - that jumps to 45-50%. That’s dangerous. You don’t want chemo flooding the fetus. Methotrexate? It barely crosses - less than 20%. That’s because the placenta doesn’t have many of the transporters it needs. But here’s the catch: even small amounts can be devastating early in pregnancy. That’s why methotrexate is absolutely avoided in the first trimester. The bottom line? There’s no one-size-fits-all. A drug that’s safe at 20 weeks might be deadly at 8 weeks. That’s why timing, dosage, and drug choice all matter.Why Animal Studies Don’t Tell the Whole Story
Most drug safety data comes from rats and mice. But their placentas are completely different. Human placental tissue has more layers, tighter barriers, and different transporter patterns. In mice, some drugs cross 3 to 4 times faster than in humans. That’s why a drug deemed “safe” in animals can still be risky in people. That’s why researchers now use human placenta models - like the “placenta-on-a-chip.” These tiny devices mimic blood flow and tissue structure. One study showed glyburide (a diabetes drug) crossed at 5.6% in the chip - almost exactly what was seen in real human tissue. That’s a big step forward. Still, most studies use term placentas. But the most critical development happens early. We’re still missing data on how drugs behave in the first 12 weeks. That’s a huge blind spot.
What the FDA and Doctors Are Doing About It
In 2015, the FDA changed how drug labels describe pregnancy risks. No more vague letters like “Category C.” Now, labels must include:- Placental transfer data
- Fetal exposure levels
- Timing of risk
- Real-world outcomes from pregnancy registries
That’s a direct result of past failures. And it’s forcing drug companies to test placental transfer earlier in development. In 2022, the European Medicines Agency said the same: evaluate placental transfer for every drug that might be used by women of childbearing age.
Still, 45% of prescription drugs have no reliable pregnancy safety data. That means doctors are often guessing. That’s why therapeutic drug monitoring is recommended for drugs with narrow safety margins - like digoxin or lithium. Even though digoxin crosses the placenta, it’s not affected by common transporter blockers. So knowing the mother’s blood level helps predict the baby’s exposure.
The Future: Targeted Therapy or New Risks?
Scientists are now designing nanodrugs that could deliver medicine directly to the fetus - say, for genetic disorders or infections. Sounds promising. But here’s the problem: nanoparticles might get stuck in the placenta. They could cause inflammation or damage. And we don’t know the long-term effects. Clinical trials are starting to test P-gp inhibitors to help deliver drugs like antibiotics or antivirals to the fetus. But if you shut down the placenta’s natural defenses, you might accidentally let in something harmful. The balance is delicate.What You Need to Know
If you’re pregnant or planning to be:- Don’t stop prescribed meds without talking to your doctor. Untreated conditions like epilepsy, depression, or high blood pressure can be more dangerous than the drugs.
- Ask: “Is this drug known to cross the placenta? What’s the evidence?”
- Timing matters. The first trimester is the most sensitive window.
- Use pregnancy registries. Many drug manufacturers track outcomes. Your data helps others.
- For chronic conditions, work with a specialist - maternal-fetal medicine, neurologist, or psychiatrist - not just your OB.
The placenta is not your enemy. It’s trying to protect your baby. But it’s not perfect. Understanding how drugs move through it isn’t just science - it’s the key to safer pregnancies.
Can I take my regular medication while pregnant?
Some medications are safe, others aren’t - and it depends on the drug, the stage of pregnancy, and your health condition. Never stop or start a drug without consulting your doctor. For example, folic acid and certain antihypertensives are generally safe, while isotretinoin and thalidomide are absolutely not. Your provider can help you weigh risks and switch to safer alternatives if needed.
Do all drugs cross the placenta equally?
No. Small, fat-soluble, non-ionized drugs like alcohol, nicotine, and many antidepressants cross easily. Large, water-soluble, or highly protein-bound drugs like insulin or warfarin barely cross. Transporters like P-gp and BCRP also actively block certain drugs, especially HIV medications and chemotherapy agents.
Why is the first trimester more dangerous for drug exposure?
In the first trimester, the placenta is still developing. Its protective transporters aren’t fully active, and the barrier between mother and baby is thinner. This is also when major organs are forming. Even small amounts of a teratogenic drug - like valproic acid or thalidomide - can cause serious birth defects during this window.
Are over-the-counter drugs safe during pregnancy?
Not necessarily. Many OTC drugs, including pain relievers like ibuprofen and cold medicines with pseudoephedrine, can cross the placenta and affect fetal development. Acetaminophen is generally considered safer for short-term use, but even that should be used at the lowest effective dose. Always check with your doctor before taking anything - even herbal supplements.
How do doctors know which drugs are safe in pregnancy?
Doctors rely on pregnancy registries, clinical studies, and updated FDA labeling. Drugs like sertraline and lamotrigine have decades of safety data from thousands of pregnancies. Others have little or no data. When evidence is limited, doctors choose the option with the best-known safety profile and monitor closely. Research continues - especially with new placental models and non-invasive imaging.


Shane McGriff
January 19, 2026 AT 03:57This is one of those posts that makes you realize how little we actually know about what's happening inside the body during pregnancy. I used to think the placenta was just a passive filter, but learning about those efflux transporters like P-glycoprotein? Mind blown. It's like the placenta has its own security team deciding who gets in. And the fact that some drugs get actively pumped back out? That's not just biology-it's warfare at a cellular level. I'm gonna start asking my OB way more questions now.
Jacob Cathro
January 20, 2026 AT 08:25lol so basically the placenta is just a bouncer with a PhD? 🤡
Also why is everyone acting like this is new info? I read this in my bio 101 textbook in 2017. We’ve known about thalidomide for 60+ years. Why are we still pretending this is cutting edge? 🙄
Paul Barnes
January 20, 2026 AT 10:13The distinction between molecular weight, lipid solubility, protein binding, and ionization is accurately described and well-structured. However, the omission of placental metabolism as a contributing factor to fetal exposure is a significant oversight. Sulfation, glucuronidation, and CYP450 activity in placental tissue can significantly alter drug bioavailability prior to fetal circulation. This deserves mention.
pragya mishra
January 21, 2026 AT 19:45I don’t care what the science says-I’ve seen too many women get told ‘it’s fine’ and then their babies are born with problems. If you’re pregnant and you’re taking anything, you’re gambling. And don’t tell me ‘folic acid is safe’-I know someone whose kid had autism after she took it. Coincidence? Maybe. But I’m not risking it.
Andy Thompson
January 23, 2026 AT 02:33THEY’RE HIDING THE TRUTH AGAIN!! 🚨
Big Pharma and the FDA don’t want you to know that 80% of these drugs are tested on rats who have PLACENTAS THAT DON’T EVEN LOOK LIKE HUMANS!! 😡
And now they’re pushing ‘nanodrugs’?? That’s how they’re going to microchip our babies!! 👶🤖
Wake up, sheeple!!
kumar kc
January 23, 2026 AT 05:50Stop taking drugs. Period. Your body was made to handle pregnancy without chemicals. If you need medication, you made poor life choices.
Thomas Varner
January 25, 2026 AT 00:27Okay, so… the placenta is basically a bouncer with a PhD in pharmacology… and it’s got a preference for fat-soluble stuff? 🤔
So… if I drank a bottle of wine at 6 weeks… it’s basically like inviting a bunch of drunk partygoers into a baby’s birthday party… and the bouncer just shrugged? 😅
Also, I now have a new favorite word: ‘efflux transporters.’ Sounds like a sci-fi villain.
Emily Leigh
January 25, 2026 AT 06:06Wow, what a shocker-science says the body is complicated. 🙄
Next you’ll tell me the sun rises in the east or that water is wet. I’ve been saying this for years: everything crosses. Everything. The ‘smart filter’ narrative is just corporate PR dressed up as biology. They want you to think there’s a ‘safe’ drug. There isn’t. Just less-bad ones.
Renee Stringer
January 26, 2026 AT 06:02It’s interesting how the article emphasizes the placenta’s protective role, yet the data shows such high transfer rates for SSRIs and opioids. I wonder if the emotional weight of neonatal withdrawal is ever discussed with patients, or if it’s just buried in footnotes.
Crystal August
January 28, 2026 AT 01:58I’m not a doctor, but I’ve read a lot of articles. And I’m pretty sure the FDA is just playing us. They approve drugs based on profit, not safety. If a drug helps rich people feel better, it gets approved-even if it kills babies. That’s capitalism. And we’re all just lab rats waiting for our turn.
Nadia Watson
January 28, 2026 AT 10:33As someone who grew up in a culture where pregnancy was seen as a time of natural purity, this article helped me reconcile modern medicine with ancestral wisdom. The placenta isn’t a wall, but it’s not a sieve either-it’s a dialogue. And we’re just learning how to listen. Thank you for sharing this with nuance. 🙏
Also, I think ‘placenta-on-a-chip’ is one of the most beautiful phrases I’ve ever read.
Courtney Carra
January 30, 2026 AT 00:34So… the placenta is basically a gatekeeper with mood swings? 😏
And the fact that it’s ‘leakier’ in the first trimester? That’s wild. I always thought the baby was ‘protected’ the whole time. Turns out, the most dangerous time is when you don’t even know you’re pregnant…
So if I had a glass of wine before I missed my period… was I already a monster? 🤔
Also, why do I feel like I need to cry now? 🥺
thomas wall
February 1, 2026 AT 00:13The ethical implications of this research are profound. The deliberate exclusion of pregnant women from clinical trials, under the guise of protection, has created a chasm of ignorance. We now rely on post-hoc data-often incomplete, sometimes misleading-to guide clinical decisions. This is not merely a scientific gap; it is a moral failure of the medical-industrial complex. We must demand inclusion, not exclusion, in research design.
clifford hoang
February 1, 2026 AT 11:25Let me tell you what they don’t want you to know…
That ‘smart filter’? It’s not natural. It’s been genetically modified by the government to control population growth. 🤫
They let in the drugs that make you docile-SSRIs, beta-blockers-but block the ones that could make you think too hard. That’s why you feel numb. That’s why you can’t quit. That’s why they’re pushing ‘nanodrugs’-to track your baby’s DNA from the womb. 📡
They’ve been doing this since the 1950s. Thalidomide? A distraction. A test. And now they’re ready to upgrade.
Check your baby’s cord blood. You’ll see the signs. I know I did.
clifford hoang
February 2, 2026 AT 13:02Wait… you said the placenta pumps drugs back out? So… if I take a supplement to boost P-glycoprotein… I could block all the drugs? Even the ones I’m supposed to be taking?
That’s insane. That means I could be poisoning my baby without even knowing it…
And what if the ‘bouncers’ get tired? What if they’re overwhelmed by too many meds? What if they’re just… lazy?
…I think I need to call my doctor.