
REMS Program Checker
Check REMS Requirements
When a drug can save your life but also carry a serious risk-like causing birth defects, sudden heart problems, or dangerous brain changes-the FDA doesn’t just slap on a warning label and call it a day. That’s where REMS programs come in. These aren’t just extra paperwork. They’re active, enforced safety systems designed to make sure high-risk medications are used safely, even if it means extra steps for doctors, pharmacists, and patients.
What Exactly Is a REMS Program?
REMS stands for Risk Evaluation and Mitigation Strategies. It’s a formal system created by the U.S. Food and Drug Administration (FDA) under the 2007 Food and Drug Administration Amendments Act. The goal? To manage serious safety risks tied to certain prescription drugs that can’t be controlled with regular labeling alone.
Think of it this way: most drugs have side effects listed on their packaging. But for some drugs-like those used to treat cancer, severe mental illness, or rare autoimmune diseases-the risks are so serious that the FDA requires more than a warning. REMS adds layers of protection: certified prescribers, mandatory patient registries, special dispensing rules, and even required monitoring tests.
It’s not about keeping drugs off the market. It’s about letting them stay on the market-safely. Drugs like isotretinoin (for severe acne), clozapine (for treatment-resistant schizophrenia), and thalidomide (for leprosy and multiple myeloma) have all been kept available because REMS makes their use manageable. Without REMS, many of these drugs would never have been approved.
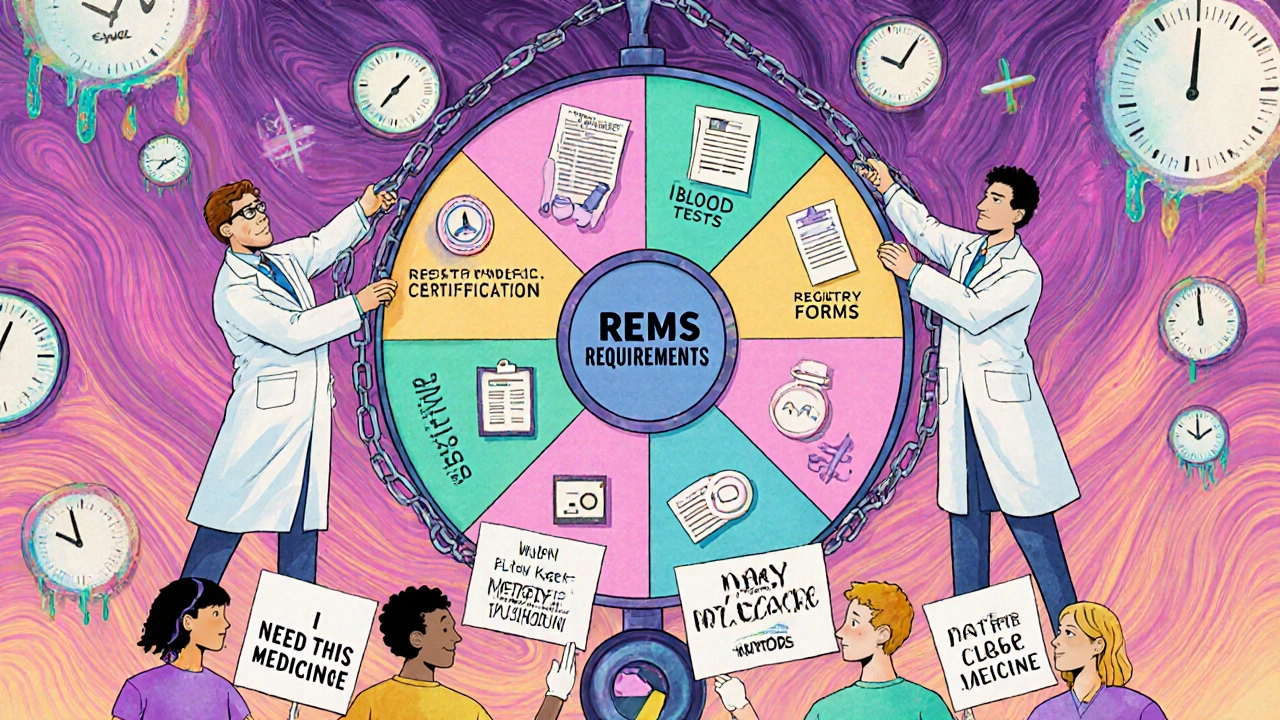
How REMS Works: The Key Elements
Not all REMS programs are the same. Each one is built around the specific danger of the drug. The FDA uses three main tools to manage risk:
- Medication Guides: These are printed handouts given to patients when they pick up their prescription. They explain the risks in plain language-no medical jargon.
- Communication Plans: These are training materials and alerts sent to doctors and pharmacists. They make sure prescribers know the red flags and how to respond.
- Elements to Assure Safe Use (ETASU): This is the strictest layer. It can include:
- Prescriber certification: Doctors must complete training and register before they can write the prescription.
- Pharmacy certification: Only approved pharmacies can dispense the drug.
- Patient enrollment: Patients must sign up in a national registry so their use is tracked.
- Special dispensing rules: The drug can only be given in a hospital or clinic, not a regular pharmacy.
- Lab monitoring: Blood tests or other checks must be done regularly-sometimes weekly.
Take Zyprexa Relprevv, an injectable antipsychotic. Because it can cause sudden sedation or delirium within minutes of injection, it can only be given in certified clinics. Patients must stay for three hours after the shot so staff can watch for reactions. That’s not a suggestion-it’s the law.
Why REMS Exists: The Balance Between Risk and Benefit
Some drugs are dangerous, but they’re also life-saving. Clozapine can help people with schizophrenia who haven’t responded to any other treatment. But it can wipe out white blood cells, leaving patients vulnerable to deadly infections. Without weekly blood tests, the risk is too high.
REMS makes that trade-off work. The FDA doesn’t require REMS for every drug. In fact, only about 5% of approved medications need it. The rest are managed with standard labeling and post-market safety monitoring.
The FDA looks at several factors before requiring a REMS:
- How severe is the potential harm?
- How common is the risk in the population?
- How serious is the condition being treated?
- Is this a new drug with unknown long-term effects?
- How many people will use it?
For example, oncology drugs make up nearly 40% of all REMS programs. That’s because targeted cancer therapies often attack healthy cells along with cancer cells, leading to serious side effects like organ damage or immune collapse. REMS ensures these drugs are only given to patients who can be monitored closely.
Who’s Responsible? The Drug Company, Not the FDA
Here’s something most people don’t realize: the FDA doesn’t run REMS programs. The pharmaceutical company that makes the drug does.
When a company applies for FDA approval, they must submit a proposed REMS plan. The FDA reviews it, approves it, or asks for changes. Once approved, the company is responsible for:
- Training prescribers and pharmacists
- Running the patient registry
- Providing educational materials
- Reporting on program effectiveness
- Paying for everything
That’s why REMS programs cost the industry over $1.2 billion a year. Some simple programs with just a medication guide cost $500,000 annually. Complex ones with registries, special pharmacies, and lab tracking can cost over $15 million per year.
The FDA audits these programs. In 2022, they issued 17 warning letters to drugmakers for failing to meet REMS requirements. One company paid a $2.1 million fine for not properly managing the clozapine REMS program.
The Real-World Impact: Delays, Frustrations, and Patient Harm
REMS saves lives-but it also slows things down.
A 2022 survey of 1,250 U.S. doctors found that 68% saw delays in starting REMS-required medications. For 42% of them, those delays hurt patient outcomes. One doctor described a patient with severe psoriasis who had to wait 10 days for a REMS-certified appointment, during which their skin lesions worsened and they developed a serious infection.
Pharmacists are on the front lines. A 2023 survey showed 73% of hospital pharmacists spend 2 to 5 extra hours per week just handling REMS paperwork. For clozapine, that means verifying weekly blood test results, checking patient registry status, and confirming pharmacy certification-all before the pill can be handed over.
The iPLEDGE program for isotretinoin (Accutane) is infamous. To get the drug, patients must:
- Take two negative pregnancy tests (for women of childbearing age)
- Complete monthly online education
- Be enrolled in the system for 30 days before receiving the first prescription
- Have their doctor and pharmacy certified
On Reddit’s r/pharmacy community, users share stories of patients waiting 3 to 7 days just to get their acne treatment. One pharmacist wrote: “I had a 17-year-old girl cry because she couldn’t get her medication before her school pictures. She’d been waiting since summer.”
Is REMS Working? Experts Are Split
There’s no denying REMS has kept dangerous drugs from causing mass harm. Dr. Robert Temple, former top FDA scientist, called it “essential for bringing critical therapies to market.”
But critics say some REMS programs are outdated, overly bureaucratic, and don’t actually improve safety.
Dr. Aaron Kesselheim from Harvard testified that the REMS for long-acting opioids required doctors to take a training course-but didn’t require them to check if patients were taking the pills correctly. He argued that didn’t reduce overdoses-it just created paperwork.
The FDA admits it too. In 2022, former Acting Commissioner Dr. Janet Woodcock said, “Not all REMS programs have been equally effective.”
And here’s the kicker: a 2023 joint FDA-PhRMA report found that 63% of REMS programs don’t even measure whether they’re working. No one knows if the extra steps are actually preventing harm-or just making life harder for patients and providers.
What’s Changing? The Future of REMS
The FDA is trying to fix the system. In 2023, they launched the REMS Integration Initiative to standardize 22 of the 78 active programs onto one digital platform. That means fewer logins, fewer passwords, and fewer errors.
They’ve also started sunsetting REMS programs when they’re no longer needed. Thalidomide’s REMS was lifted in August 2023-after 20 years-because the risks are now well understood and managed through standard practices.
And they’re going digital. Pilot programs are testing smartphone apps to track patients taking anticoagulants. Instead of weekly blood draws, patients might use a connected device that sends lab results directly to their doctor.
By 2027, nearly half of all new cancer drugs will likely need REMS. But the goal now isn’t just to add more programs-it’s to make them smarter, faster, and less burdensome.
What Patients and Providers Should Do
If you’re prescribed a REMS drug:
- Ask your doctor: “Is this drug under a REMS program?”
- Find out what steps you need to take-registration, tests, clinic visits.
- Start the process early. REMS delays can be weeks long.
- Keep copies of all paperwork. Pharmacies often need them to verify eligibility.
If you’re a prescriber or pharmacist:
- Check the FDA’s REMS Dashboard regularly for updates.
- Use the new integrated platforms where available-they reduce errors.
- Advocate for patients. If a REMS requirement doesn’t improve safety, say so.
REMS isn’t perfect. But it’s the best system we have for balancing the power of dangerous drugs with the need to protect people. The challenge now isn’t to get rid of it-it’s to make it work better for everyone.
What drugs require a REMS program?
Drugs with serious safety risks that can’t be managed with standard labeling require REMS. Examples include isotretinoin (for acne), clozapine (for schizophrenia), thalidomide (for leprosy and cancer), and many cancer therapies like bortezomib or lenalidomide. As of October 2023, 78 REMS programs cover about 150 medications, mostly in oncology, neurology, and immunology.
Who pays for REMS programs?
The pharmaceutical company that makes the drug pays for everything: training, registries, patient materials, pharmacy certification, and system maintenance. The FDA doesn’t fund REMS. Industry spending on REMS totaled $1.2 billion in 2022, with some programs costing over $15 million annually.
Can a REMS program be removed?
Yes. The FDA can remove or modify a REMS if new data shows the risks are better managed through standard practices. In August 2023, the REMS for thalidomide was officially sunset after 20 years because improved education and monitoring made the program unnecessary.
Why do REMS programs cause delays in getting medication?
REMS adds steps: prescriber certification, patient registration, lab verification, pharmacy checks, and sometimes mandatory observation periods. Each step requires time and coordination. For example, the iPLEDGE program for isotretinoin requires two pregnancy tests, monthly education, and a 30-day enrollment period-adding days or weeks to treatment start times.
Are REMS programs the same as the EU’s Risk Management Plans?
No. In the European Union, all new medicines require a Risk Management Plan (RMP). In the U.S., REMS is only required for drugs with serious, specific risks. REMS is more targeted and less universal than RMPs. The FDA uses REMS selectively, while the EU applies similar frameworks to nearly every new drug.





Austin Levine
October 29, 2025 AT 16:24REMS is one of those things that seems ridiculous until you realize how many people it’s actually saved. I’ve seen patients on clozapine go from suicidal to stable because of the weekly blood tests. Yeah, it’s a pain, but it’s not bureaucracy for bureaucracy’s sake.
Matthew King
October 29, 2025 AT 17:57bro i got my accutane prescription and it took 3 weeks just to get the damn thing. had to do 2 pregnancy tests, watch a 45-min video, fill out 7 forms, and then my pharmacy had to call the FDA portal. i’m 19 and my acne isn’t even that bad. why does this feel like a cult?
Andrea Swick
October 30, 2025 AT 12:02It’s interesting how REMS programs evolved from emergency measures into institutionalized processes that outlive their original purpose. Some of these requirements were designed for drugs that are now well-understood, yet we still force patients and providers through the same hoops. The system hasn’t kept pace with data collection or digital tools. We’re managing risk with paper forms in a world that runs on APIs. It’s not that REMS is bad-it’s that we stopped asking if it’s still the best way.
Amelia Wigton
November 1, 2025 AT 07:57Let’s be clear: REMS is a regulatory overreach disguised as patient safety. The iPLEDGE program is a nightmare of compliance theater. Mandatory monthly online modules? For acne? The FDA has no business micromanaging dermatology. And don’t get me started on the $15M-per-year costs-those are passed on to patients via higher drug prices. This isn’t risk mitigation; it’s liability armor for pharma.
Joe Puleo
November 2, 2025 AT 09:35If you’re on a REMS drug, just start the paperwork early. Seriously. My cousin needed clozapine and waited six weeks because she didn’t know she had to get her blood work done before her prescriber could even certify. Once she figured it out, it was smooth sailing. Don’t wait till the last minute-it’ll save you stress and maybe even a hospital visit.
Keith Bloom
November 3, 2025 AT 03:08lol the FDA thinks they’re saving lives but really they’re just protecting pharma from lawsuits. You think a 17-year-old girl crying over acne meds is tragic? Nah, it’s just the cost of corporate liability. And those ‘safety’ training modules? Half the docs skip ‘em. The system’s a joke. Also, why do i keep seeing ‘REMS’ spelled ‘REMS’? It’s not an acronym anymore-it’s a brand.
Ben Jackson
November 4, 2025 AT 17:49REMS is a textbook example of ‘good intention, poor execution.’ The framework is sound-risk stratification, tiered controls, monitoring-but the implementation is fragmented, analog, and siloed. We need a unified digital platform, not 78 different portals with different logins. Pharma should be incentivized to build interoperable systems, not just check boxes. The FDA’s 2023 integration push? Long overdue.
Bhanu pratap
November 5, 2025 AT 16:01As someone from India, I’ve seen how the U.S. system struggles with REMS-but I also know what happens without it. In my home country, drugs like thalidomide were once sold over the counter. People lost children because no one monitored. REMS is frustrating, yes-but it’s also a lifeline. We need to fix the delays, not the concept. Let’s digitize, not dismantle.
Meredith Poley
November 6, 2025 AT 02:15Let me get this straight: we spend $1.2 billion a year so pharmacists can verify pregnancy tests for acne treatment, but we can’t fix the opioid crisis because the REMS for long-acting opioids didn’t require pill counts? Brilliant. The FDA is like a guy who locks the front door but leaves the window wide open. And you call that risk management?
Mathias Matengu Mabuta
November 7, 2025 AT 06:14It is not within the purview of any regulatory agency to impose such onerous, non-evidence-based, and constitutionally suspect administrative burdens upon the private exercise of medical autonomy. The REMS paradigm constitutes a de facto prior restraint upon the physician-patient relationship, and the financial exactions levied upon pharmaceutical manufacturers are, in effect, an unconstitutional taking under the Fifth Amendment. The FDA’s assertion of authority is predicated upon a misinterpretation of the FDAAA, and the judicial deference afforded to agency discretion has reached its logical terminus.
Ikenga Uzoamaka
November 8, 2025 AT 05:56REMS is just another way for rich countries to control poor people’s health… I saw a woman in Lagos die because she couldn’t get her cancer drug-no REMS, no clinic, no nothing. But here? You need 7 forms to get acne cream. This is not safety. This is privilege. And the FDA? They don’t care about us. They care about lawsuits.
Lee Lee
November 8, 2025 AT 21:29Think about it: REMS is just the tip of the iceberg. The FDA doesn’t want you to know this, but every REMS program is secretly tied to a pharmaceutical lobbying contract. The $15 million cost? That’s not for safety-it’s for the pharma company to buy silence from the FDA. The thalidomide REMS was sunset not because it was safe-but because the patent expired and they didn’t need to control distribution anymore. Wake up. This isn’t public health. It’s corporate control.