Every time you pick up a bottle of generic ibuprofen or metformin at the pharmacy, you’re holding the end result of a complex, global journey. It’s not just a pill. It’s a chain of raw materials, factories, regulators, distributors, and negotiators - all working to get an affordable medicine into your hands. And while the process sounds simple, the reality is anything but.
Where It Starts: The Raw Materials
It begins with something invisible: Active Pharmaceutical Ingredients, or APIs. These are the actual chemical compounds that make a drug work. For example, the API in generic atorvastatin is what lowers cholesterol. But here’s the catch: 88 percent of all API manufacturing happens outside the United States, mostly in China and India. The U.S. produces just 12 percent of its own essential drug ingredients. This global setup wasn’t always the norm. Before the 1990s, most APIs were made domestically. But as labor and production costs rose, manufacturers moved overseas. It saved money - but also created vulnerability. During the pandemic, when factories in India shut down or shipping slowed, 170 different generic drugs faced shortages. The FDA saw this coming. They ramped up inspections of foreign facilities from 248 in 2010 to 641 in 2022. Still, monitoring thousands of factories across continents is a massive challenge.The Approval Hurdle: FDA and ANDA
Once the API is made, it’s shipped to a drug manufacturer - often in the U.S., Europe, or another regulated region. But they can’t just start packaging and selling. First, they must prove their generic version is identical in effect to the brand-name drug. That’s done through an Abbreviated New Drug Application, or ANDA. The FDA doesn’t require new clinical trials. Instead, manufacturers must show their product has the same active ingredient, strength, dosage form, and route of administration. More importantly, they must prove it’s bioequivalent - meaning it gets into your bloodstream at the same rate and amount as the brand. This is where quality control kicks in. Every batch is tested for purity, potency, and stability under Good Manufacturing Practices (GMP). One failed test can delay a product launch by months. The approval process can take 1-3 years. But once approved, the manufacturer can start selling. And here’s where the real money game begins.Who Gets Paid: The 36 Percent Problem
You might think the company that makes the generic pill takes home the biggest slice of the pie. They don’t. According to research from the University of Southern California’s Schaeffer Center, generic manufacturers capture only 36 percent of the total money spent on generic drugs. The rest? Goes to distributors, pharmacies, PBMs, and other middlemen. Compare that to brand-name drugs, where manufacturers keep 76 percent of the spending. Why the gap? Because generic drugs compete on price - fiercely. There’s no patent protection. Dozens of companies can make the same drug. So manufacturers slash prices to win contracts. Some sell APIs for pennies. A 10 mg tablet of atorvastatin might cost a manufacturer 2 cents to produce. But by the time it hits your pharmacy shelf, the price has climbed - not because of the maker, but because of everyone else in the chain.
The Middlemen: Wholesalers and PBMs
After manufacturing, the drugs go to wholesale distributors like McKesson, AmerisourceBergen, and Cardinal Health. These companies buy in bulk and sell to pharmacies. They don’t just deliver - they negotiate. They offer “prompt payment discounts” to manufacturers who pay quickly. In return, pharmacies get lower prices. But the real power lies with Pharmacy Benefit Managers, or PBMs. CVS Caremark, OptumRX, and Express Scripts control about 80 percent of the U.S. PBM market. They’re the invisible middlemen between insurers, pharmacies, and drugmakers. Their job? Negotiate rebates, set formularies, and decide which drugs get covered. Here’s the twist: Generic manufacturers rarely pay rebates to PBMs. Unlike brand-name companies that offer huge discounts to get on a formulary, generics compete on price alone. So PBMs don’t negotiate with them - they set reimbursement limits instead.How Pharmacies Get Paid: MAC Pricing
When you fill a prescription, your insurance doesn’t pay the full price. Instead, it uses something called Maximum Allowable Cost, or MAC. This is a fixed reimbursement rate set by the PBM for each generic drug - say, $2.50 for a 30-day supply of lisinopril. The problem? Many pharmacies buy that same bottle for $3 or more. That’s a loss. A 2023 survey by the American Pharmacists Association found that 68 percent of independent pharmacies are selling generics below cost because MAC rates haven’t kept up with rising wholesale prices. Pharmacies are stuck between a rock and a hard place: if they refuse to fill the prescription, patients go elsewhere. If they fill it, they lose money. This is why large pharmacy chains - like CVS or Walgreens - have more leverage. They buy in huge volumes and negotiate better deals with wholesalers. Independent pharmacies? They often join buying groups just to survive.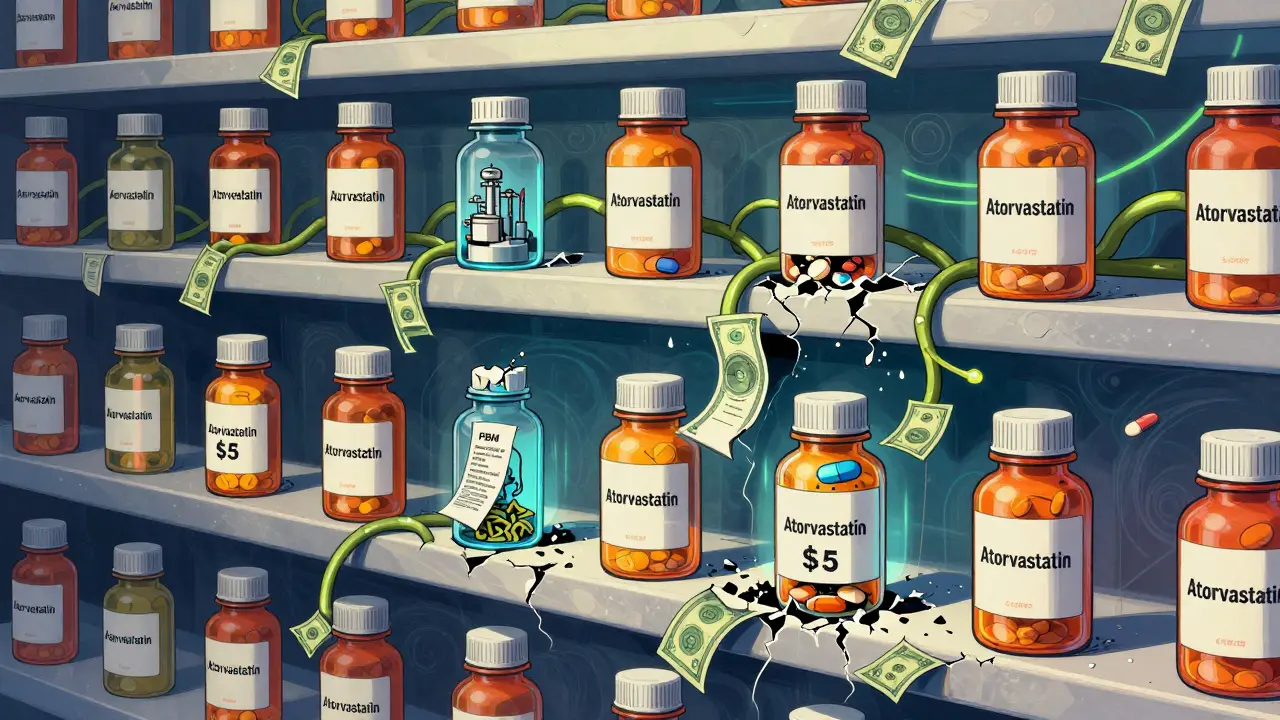





Moses Odumbe
December 19, 2025 AT 13:1388% of APIs come from India and China? No wonder we had shortages during the pandemic. 🤯 I mean, imagine if your phone’s battery only came from one country - you’d panic if that country had a strike. Same thing here. We’re one global crisis away from running out of blood pressure meds. 😬
Meenakshi Jaiswal
December 19, 2025 AT 22:31As someone who works in pharma manufacturing in India, I can tell you - we’re not cutting corners. The labs are clean, the tech is advanced. But yes, the margins are razor-thin. If you want affordable medicine, you have to accept that the people making it are barely breaking even. 💪
bhushan telavane
December 21, 2025 AT 15:37India makes 40% of the world’s generics. We’re not just factories - we’re the unsung heroes of global health. But now everyone wants to move production elsewhere. Cool. Try making a 2-cent tablet with 100% local labor and see how long you last. 😅
Mahammad Muradov
December 23, 2025 AT 06:09Let’s be honest - the U.S. got lazy. We outsourced our medicine because it was cheaper. Now we cry when it breaks. If you want national security, build your own API plants. Stop pretending this is a supply chain issue - it’s a moral failure.
Connie Zehner
December 24, 2025 AT 03:00OMG I JUST REALIZED WHY MY LISINOPRIL COSTS $5 WHEN IT COSTS 2 CENTS TO MAKE?? 😭 I’M SO ANGRY. WHO DO I COMPLAIN TO?? PBMs?? I’M SENDING THEM A LETTER WITH A CANDLE AND A THERAPY BILL. 🕯️💸
holly Sinclair
December 24, 2025 AT 16:26It’s fascinating how we’ve engineered a system where the cheapest product becomes the most opaque. The pill itself is a scientific marvel - identical in bioequivalence, purity, and dosage - yet the economic value attached to it is a labyrinth of hidden fees, rebates, and negotiated illusions. We’ve turned medicine into a derivative asset, not a human right. The tragedy isn’t the cost - it’s that we’ve stopped asking why we accept this as normal.
Monte Pareek
December 24, 2025 AT 23:09Look I’ve been in this game for 20 years and let me tell you nobody cares about transparency because nobody wants to see the sausage being made. PBMs don’t care. Pharmacies don’t care. Patients don’t care until they can’t afford their meds. The system is rigged but it’s rigged because we let it be. The answer isn’t more regulation - it’s more accountability. Name names. Expose contracts. Make the middlemen sweat. And if you’re a pharmacist selling at a loss - you’re not a martyr you’re a pawn. Speak up or get out.
Kelly Mulder
December 26, 2025 AT 05:50It is, quite frankly, an abysmal state of affairs that the United States of America - a nation of unparalleled technological and scientific prowess - has ceded the production of its most essential pharmaceuticals to foreign jurisdictions with questionable regulatory oversight. The erosion of domestic manufacturing capacity is not merely an economic misstep - it is a national security vulnerability of the highest order. One must question the wisdom of a populace that prioritizes marginal cost savings over existential resilience.
Tim Goodfellow
December 27, 2025 AT 11:07Blockchain for pills? AI predicting shortages? Dude that’s next-level stuff. Imagine a world where every pill has a digital passport - where you scan it and see: Made in Hyderabad, shipped via Maersk, cleared by FDA on 3/14, batch #G2024-001. That’s not sci-fi - that’s just good hygiene. We’re in the Stone Age of drug tracking. Time to upgrade.
Elaine Douglass
December 29, 2025 AT 00:44my pharmacist told me last week that some generics are actually made in the same factory as the brand name just under a different label. i didnt even know that. makes you wonder why we pay more for the brand. also i just started buying my meds from costco and saved like 70%. weird but true.
Takeysha Turnquest
December 29, 2025 AT 18:13we are not patients we are consumers we are not healing we are transactioning we are not trusting we are negotiating we are not alive we are optimizing
Emily P
December 29, 2025 AT 21:00Is there any public database where I can see the actual manufacturing cost of a generic drug? I tried looking but everything’s locked behind corporate NDAs. Just curious if anyone’s found a way to access that data.
Vicki Belcher
December 31, 2025 AT 15:11Thank you for writing this. 💙 I’m so glad someone finally explained why my $3 insulin costs $50 at the pharmacy. It’s not the drug - it’s the system. And guess what? We can fix it. One transparent contract, one fair MAC rate, one honest conversation at a time. You’re not alone. 🌱