
Most people think of MRSA as a hospital problem - something that happens to patients after surgery or during a long stay. But that’s not the whole story. Since the late 1990s, a new kind of MRSA has been spreading in gyms, prisons, dorms, and homes - among people who’ve never set foot in a hospital. This strain isn’t just different in where it shows up. It’s different in how it behaves, how it spreads, and how it responds to treatment. The line between hospital MRSA and community MRSA is fading fast, and that’s changing everything about how we fight it.
What Makes MRSA So Hard to Treat?
MRSA stands for methicillin-resistant Staphylococcus aureus. It’s a type of staph bacteria that won’t die when you hit it with common antibiotics like penicillin, amoxicillin, or methicillin. That’s not a small problem - it’s a big one. These drugs used to kill staph infections in minutes. Now, they’re useless. And because MRSA doesn’t respond to the go-to antibiotics, doctors have to use stronger, more expensive ones - if they work at all.
What makes MRSA dangerous isn’t just resistance. It’s how fast it spreads and how aggressive it can be. Some strains produce a toxin called Panton-Valentine leukocidin (PVL). This toxin kills white blood cells, turning a simple skin boil into a life-threatening abscess or even a fast-moving pneumonia. In healthy young people with no medical history, this toxin is often the reason MRSA turns deadly.
Community MRSA vs. Hospital MRSA: The Genetic Divide
Not all MRSA is the same. Genetically, they’re two different animals.
Community-associated MRSA (CA-MRSA) carries a small piece of DNA called SCCmec type IV or V. This tiny genetic package doesn’t carry many resistance genes - which means it’s not resistant to dozens of drugs. But it’s packed with virulence genes. That’s why CA-MRSA causes nasty skin infections, abscesses, and sometimes necrotizing pneumonia. The most common strain in the U.S. is USA300. It’s responsible for about 70% of community cases. And here’s the kicker: 96% of CA-MRSA strains are still sensitive to clindamycin. That’s a critical detail for treatment.
Hospital-associated MRSA (HA-MRSA), on the other hand, carries much larger SCCmec types (I, II, or III). These are like genetic toolkits full of resistance genes. HA-MRSA resists not just methicillin, but often erythromycin (98% resistant), clindamycin (65% resistant), and fluoroquinolones (92% resistant). It’s built to survive in hospitals where antibiotics are used constantly. But it’s less aggressive in healthy people. It doesn’t usually cause skin infections unless the person is already weak - like someone on dialysis, with a catheter, or recovering from surgery.
How Do You Catch It? Transmission Is Not What You Think
CA-MRSA spreads through skin-to-skin contact. Think wrestling teams, locker rooms, shared towels, or even hugging someone with an undiagnosed boil. Crowded places make it worse. Military barracks? 12 times more likely to spread. Prisons? Nearly 15 times more. Homeless shelters? Almost 9 times. Injecting drug users are a major hidden reservoir - needle sharing, poor hygiene, and skin damage from repeated punctures create perfect conditions for USA300 to thrive.
HA-MRSA spreads differently. It’s often carried on the hands of healthcare workers, on bed rails, on IV lines, or on surgical tools. It infects people with broken skin - catheters, surgical wounds, breathing tubes. But here’s the twist: people are moving between hospitals and communities every day. Nurses go home. Patients get discharged. Visitors come and go. And MRSA goes with them.
Recent data from Canada shows that 27.6% of MRSA infections that started in the hospital were actually caused by community strains. And 27.5% of community infections were caused by hospital strains. That’s not a glitch - it’s the new normal. The old idea that CA-MRSA stays in the community and HA-MRSA stays in the hospital? It’s outdated.

How Do the Infections Look and Feel?
Both types often start as a red, swollen, painful bump - like a spider bite or a pimple that won’t go away. But the differences show up fast.
CA-MRSA infections are usually skin and soft tissue: boils, abscesses, cellulitis. They come on quickly. People are otherwise healthy. They don’t have catheters or recent surgery. Hospital stays? Most last under three days. Many don’t even need antibiotics - just draining the abscess and keeping it clean.
HA-MRSA infections are more likely to be deeper and more complex: bloodstream infections, pneumonia, surgical site infections, or infections around catheters. Patients are often older, sicker, or immunocompromised. Their hospital stays are longer - sometimes weeks. They’re more likely to need IV antibiotics and intensive care.
But here’s where it gets messy: a person with CA-MRSA might end up in the hospital for an abscess, and then pick up HA-MRSA while they’re there. Or a hospital patient might go home with HA-MRSA and pass it to their kids. The strains are mixing. The old labels are breaking down.
Treatment: One Size Doesn’t Fit All
If you have a skin abscess and you’re healthy, chances are it’s CA-MRSA. The best treatment? Drain it. That’s it. Antibiotics aren’t always needed. But if you do need them, clindamycin, trimethoprim-sulfamethoxazole, or doxycycline are the go-to options. They work because CA-MRSA hasn’t built resistance to them yet.
But if you’re in the hospital with a fever, low blood pressure, and an infected wound? That’s likely HA-MRSA. You’ll probably need vancomycin, daptomycin, or linezolid - drugs that are stronger, more expensive, and harder on the body. These are last-resort antibiotics. Overuse leads to resistance. And now, we’re seeing hybrid strains - CA-MRSA’s virulence with HA-MRSA’s resistance. These are the nightmares doctors fear.
Here’s the problem: if you’re a doctor treating a skin infection in the ER and you assume it’s CA-MRSA, but it’s actually a new hybrid strain with HA-MRSA resistance, your clindamycin might fail. And if you assume it’s HA-MRSA and give vancomycin to a healthy person with a simple boil, you’re overtreating - and helping resistance grow.
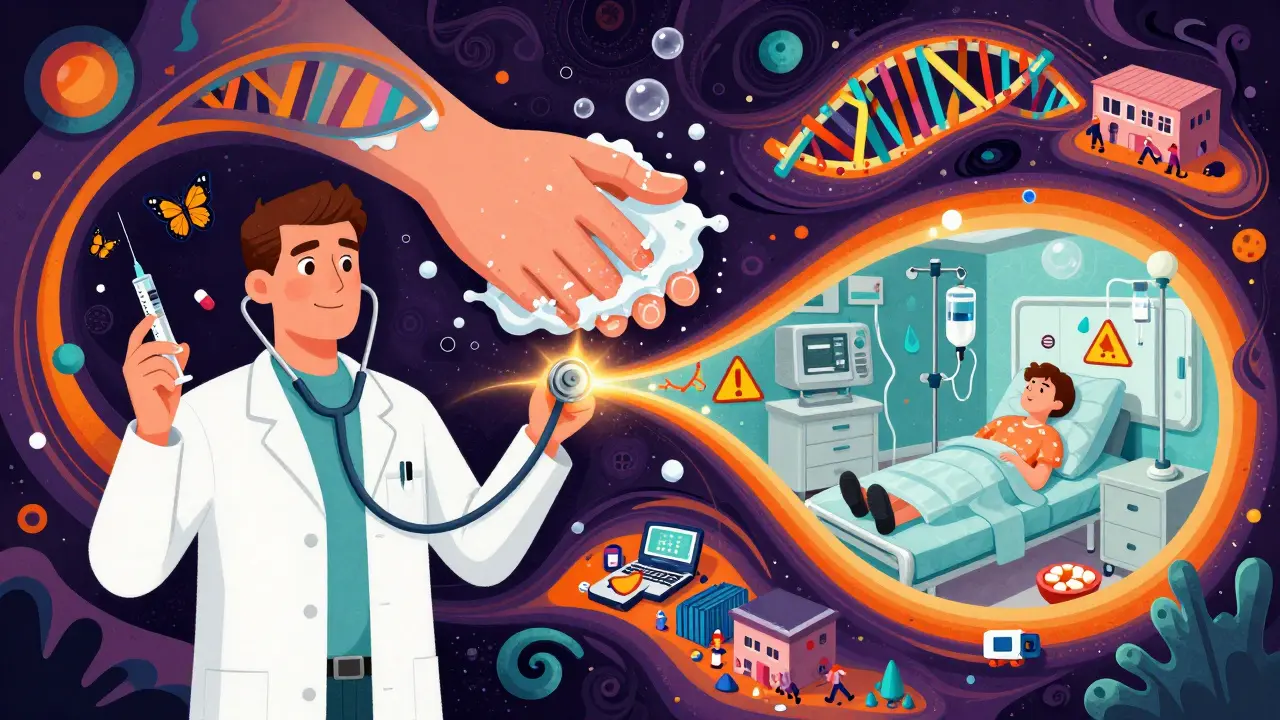
The Future: No More Separate Worlds
Experts now say we need to stop thinking of MRSA as two separate problems. It’s one problem with two faces. Surveillance systems that track only hospital cases or only community cases are missing half the picture. We need systems that follow MRSA across the entire continuum - from the prison cell to the ICU bed.
Some places are already adapting. Hospitals are screening not just surgical patients, but anyone coming in from high-risk community settings. Clinics are testing for PVL toxin to quickly identify CA-MRSA. And public health agencies are pushing for better hygiene in prisons, shelters, and gyms - not just in hospitals.
But the biggest change? Doctors are learning to treat based on the patient, not the label. A young athlete with a swollen leg? Treat for CA-MRSA. An elderly man with a catheter and fever? Treat for HA-MRSA. But if the patient just got out of prison and now has pneumonia? Treat for both.
What You Can Do
You don’t need to live in fear. But you do need to be smart.
- Wash your hands often - especially after touching shared equipment or public surfaces.
- Don’t share towels, razors, or athletic gear.
- Cover any cuts or scrapes with clean bandages until they heal.
- If you have a boil that’s red, hot, painful, or growing - see a doctor. Don’t pop it yourself.
- If you’ve been in a hospital or prison recently and you get sick, tell your doctor. That history matters.
MRSA isn’t going away. But we’re learning how to fight it - not by pretending it’s one thing, but by understanding it’s two things that are becoming one.
Is MRSA always dangerous?
No. Many people carry MRSA on their skin without ever getting sick. It only becomes dangerous when it enters the body through a cut, wound, or medical device. Healthy people with skin infections often recover with simple drainage. But for those with weakened immune systems, MRSA can lead to serious bloodstream or lung infections.
Can you get MRSA from a hospital even if you’ve never been inside one?
Yes. A growing number of community infections are caused by HA-MRSA strains that spread from patients, visitors, or healthcare workers who carry the bacteria. Even if you’ve never been hospitalized, someone you know might have brought it home. This is why the line between community and hospital MRSA is disappearing.
Why is clindamycin effective against CA-MRSA but not HA-MRSA?
CA-MRSA strains usually carry fewer resistance genes - they’re designed to be aggressive, not resistant. Clindamycin works against them because they haven’t evolved defenses against it. HA-MRSA, however, has been exposed to many antibiotics in hospitals and has picked up genes that block clindamycin. About 96% of CA-MRSA is still sensitive to it, but only 35% of HA-MRSA responds.
Does hand sanitizer kill MRSA?
Alcohol-based hand sanitizers (at least 60% alcohol) can reduce MRSA on skin, but they’re not as reliable as soap and water - especially if your hands are visibly dirty or greasy. For MRSA, washing with soap and water for at least 20 seconds is the gold standard. Sanitizers are good for quick cleanups, but not replacements.
Are there new treatments being developed for MRSA?
Yes. Researchers are testing new antibiotics, phage therapy (using viruses that target bacteria), and even vaccines. Some new drugs like ceftaroline and omadacycline are already approved and show promise against resistant strains. But the real breakthrough will come from better prevention - stopping transmission before it starts, not just treating it after.



Doreen Pachificus
January 4, 2026 AT 15:50Interesting breakdown. I never realized how much the genetics differ between strains. Makes me wonder how many other superbugs are hiding in plain sight with similar dual identities.
Dee Humprey
January 5, 2026 AT 20:17Wash your hands. Don’t share towels. Cover cuts. These are the real MVPs. No fancy drugs needed if you just stop the spread before it starts.
Shanna Sung
January 5, 2026 AT 23:54They’re lying about the real cause. This isn’t about bacteria-it’s about the CDC pushing pharmaceutical agendas. Clindamycin works? Sure… until they pull it off the market next year.
Jason Stafford
January 7, 2026 AT 21:56Let me tell you something they don’t want you to know. The government knew about this strain switch years ago. They buried the data because if people realized MRSA was spreading through gyms and prisons, they’d panic. And panic means lawsuits. And lawsuits mean they’d have to shut down half the prisons and shut down every damn CrossFit box in America. This is a controlled collapse.
USA300? That’s not a strain. It’s a bioweapon that escaped from Fort Detrick. The same place that gave us the flu vaccine that gave people Parkinson’s. You think that’s coincidence? Wake up.
I’ve seen it with my own eyes. My cousin got a boil after lifting weights. Two days later, he was in a coma. The hospital said it was MRSA. I looked up the strain code. It matched a classified 2012 DARPA report. They called it ‘Project Sweat.’
And now they’re pushing ‘hybrid strains’ like it’s new? No. They’ve been breeding them since 2008. The real reason they’re changing treatment guidelines? They’re testing new antibiotics on the public. And you’re the lab rat.
Don’t trust the CDC. Don’t trust your doctor. Don’t trust the ‘hygiene tips.’ They’re just distracting you from the truth. The bacteria isn’t the enemy. The system is.
Justin Lowans
January 9, 2026 AT 16:09This is one of the clearest, most nuanced explanations of MRSA I’ve ever read. The distinction between CA-MRSA and HA-MRSA isn’t just academic-it’s life-saving. I’ve seen patients misdiagnosed because providers defaulted to hospital protocols for otherwise healthy individuals. The shift toward patient-centered, context-aware treatment is long overdue.
And the point about transmission blurring between settings? Absolutely critical. We need to stop treating healthcare and community health as separate silos. A nurse who works the night shift at a hospital and coaches her kid’s soccer team on weekends is a vector. So is a student who gets a piercing and then shares a towel in the dorm. Our systems must adapt to the human reality, not the textbook ideal.
Thank you for highlighting the importance of PVL toxin and clindamycin sensitivity. These aren’t just facts-they’re tools that can prevent unnecessary hospitalizations and antibiotic overuse. This is public health writing at its best.
Cassie Tynan
January 11, 2026 AT 12:49So let me get this straight-we’ve got a superbug that’s basically the Avengers of bacteria: part Hulk (virulent), part Iron Man (resistant), and it’s running around in gyms and prisons like it’s auditioning for a Marvel movie? And the doctors are just now realizing they need to stop using the same playbook for a villain that changed its costume?
Classic. We spend billions on new drugs while ignoring the fact that the real problem is humans being humans. Sharing towels. Not washing hands. Getting piercings in basements. Meanwhile, the CDC is out here writing pamphlets like we’re all 8-year-olds who need a sticker chart.
Here’s a radical idea: what if we just told people to stop being gross? No fancy antibiotics. No surveillance systems. Just… don’t touch your face after you’ve touched a shared dumbbell.
saurabh singh
January 12, 2026 AT 21:18As someone from India, I’ve seen this play out in rural clinics and urban hospitals alike. In my village, MRSA was called ‘the boil that won’t heal’-no one knew the name, but everyone knew the fear. We didn’t have antibiotics, so we used turmeric paste and neem leaves. Funny thing? It worked better than some hospital treatments.
But now, with migration and travel, the strains are crossing borders like visas. My nephew got infected in Dubai after a gym session, and the strain was USA300. He didn’t even know what MRSA was. That’s the new global reality.
We need to stop treating this like a Western problem. In India, we’ve got community MRSA in slums where 15 people share one tap. But we also have HA-MRSA in private hospitals using antibiotics like candy. The solution isn’t just medical-it’s cultural. Hygiene education must be as common as Bollywood songs.
And yes, handwashing works. But only if you teach it with love, not fear. My aunt taught me to wash hands by singing a 30-second song. Now my whole family does it. Simple. Human. Effective.
Allen Ye
January 13, 2026 AT 10:19The fundamental error in contemporary medical discourse is the assumption that biological entities can be neatly categorized by geography or institutional setting. MRSA is not two strains-it is one organism exhibiting phenotypic plasticity in response to selective pressures, and the artificial dichotomy between community and hospital is a relic of reductionist epidemiology that fails to account for the dynamic flux of human mobility, antibiotic exposure, and microbial evolution.
The SCCmec cassette is not merely a genetic marker; it is a signature of evolutionary trade-offs: CA-MRSA sacrifices broad resistance for virulence, optimizing for transmission in low-antibiotic environments, while HA-MRSA embodies the cost of survival in high-antibiotic milieus-its genome bloated with resistance genes, its virulence attenuated. This is not a flaw-it is Darwinism in action.
And yet, we persist in labeling, in compartmentalizing, in treating the symptom of the system rather than the system itself. The real crisis is not MRSA-it is our inability to perceive microbial life as a continuum, a spectrum of adaptation, not a binary. We are not fighting bacteria. We are fighting the illusion of control.
When a nurse returns home from ICU to her toddler’s daycare, she is not a vector. She is a bridge. When a prisoner is released into a community without screening, he is not a threat-he is a conduit of evolutionary history. The solution is not surveillance. It is humility. We must stop trying to classify the unclassifiable and begin to coexist with the inevitable.
Jay Tejada
January 14, 2026 AT 17:41Man, I got MRSA once after a tattoo. Doc gave me clindamycin. I thought I was gonna die. Turned out I just needed the abscess drained and some rest. No big hospital drama. But my cousin? He got it after knee surgery and ended up on vancomycin for weeks. Same bug, different worlds.
They say it’s getting worse. I say it’s just getting noticed. We’ve always had these bugs. We just didn’t have the tech to tell them apart.
Siobhan Goggin
January 15, 2026 AT 05:16I work in a GP clinic and see this all the time. A teenager comes in with a red bump-looks like a spider bite. We drain it, no antibiotics. Three weeks later, same kid’s dad comes in with pneumonia after a hospital visit for his back surgery. Same strain. Different story. It’s all connected now.
Vikram Sujay
January 16, 2026 AT 18:53The convergence of community and hospital strains reflects not merely a biological phenomenon but a profound sociological shift in human interaction patterns. The erosion of boundaries between institutional and domestic spheres-facilitated by mobility, urban density, and the normalization of shared environments-has rendered the traditional epidemiological taxonomy obsolete.
It is not sufficient to classify pathogens by origin; we must now classify human behavior as a vector. The act of sharing a towel, the frequency of handwashing, the cultural norms surrounding wound care-these are the true determinants of transmission dynamics.
Therefore, the imperative is not solely technological but ethical: to cultivate collective responsibility not as a policy directive, but as a social virtue.
mark etang
January 17, 2026 AT 10:27As a public health official, I commend this comprehensive overview. The integration of genetic, clinical, and behavioral data into a unified surveillance framework is no longer optional-it is essential. We are developing a real-time MRSA tracking dashboard that links hospital admissions with community exposure histories. Early results show a 40% increase in cross-transmission events when both data streams are combined. This is the future of infection control.
Ethan Purser
January 17, 2026 AT 14:44We’re all just meat sacks with bacteria floating around inside us, waiting for a crack in the armor. The real question isn’t whether MRSA is hospital or community-it’s why we keep giving it a reason to strike. We’re all just one unwashed hand, one unbandaged cut, one arrogant assumption away from becoming a statistic.
Doctors treat the bug. But who’s treating the human? Who’s asking why someone’s living in a dorm with 12 guys and no soap? Why someone’s getting tattoos in a basement with reused needles? Why someone’s getting surgery in a hospital where the sinks haven’t been cleaned since 2019?
MRSA isn’t the monster. We are. We built the conditions. We ignored the warnings. We thought antibiotics were magic. Now we’re just cleaning up the mess we made while pretending we didn’t know it was coming.
And the worst part? We’ll do it again. With the next bug. Because we never learn. We just get better at naming things.
josh plum
January 19, 2026 AT 08:40Look, if you’re not washing your hands after you touch a gym machine, you deserve to get MRSA. And if you’re sharing a razor with your buddy because you ‘trust him’-congrats, you’re a walking biohazard. This isn’t a conspiracy. It’s basic hygiene. But people today think they’re too cool to wash their hands. So now they’re dying in the ER like it’s a TikTok trend.
And don’t even get me started on the ‘natural remedies’ crowd. Turmeric paste? Please. I’ve seen people with abscesses the size of baseballs because they ‘trusted the internet’ instead of a doctor. You want to be a hero? Wash your damn hands.
Jason Stafford
January 19, 2026 AT 12:16You think this is about hygiene? No. It’s about control. The CDC doesn’t want you to know that CA-MRSA can be stopped with a $3 bottle of tea tree oil and a clean towel. They want you to believe you need a prescription. They want you dependent. They want you afraid. That’s how they make money. That’s how they stay in power.
And now they’re calling it a ‘hybrid strain’? That’s not science. That’s marketing. It’s a new word to sell a new drug. I’ve seen the internal memos. They’ve been pushing this narrative since 2017. The real solution? Stop going to gyms. Stop going to hospitals. Stop trusting the system.
And if you’re reading this and you’re healthy? You’re one of the lucky ones. For now.